Apoenzyme: what it is, characteristics and chemistry of its functioning.
Let's see what an apoenzyme is, what its chemical properties are and how it works.
From a biochemical point of view, a metabolic pathway or metabolic pathway is a succession of chemical reactions in which a substrate is transformed and gives rise to a product.Apoenzyme: what it is, characteristics and chemistry of its functioning.
Let's see what an apoenzyme is, what its chemical properties are and how it works.
From a biochemical point of view, a metabolic pathway or metabolic pathway is a succession of chemical reactions in which a substrate is transformed and gives rise to a final product/products, through a series of intermediary metabolites. Metabolic pathways occur continuously in all cells of our body because, for example, glycolysis is an essential chemical process for cells to obtain energy. Metabolic pathways are essential to maintain our homeostatic equilibrium, i.e. that each and every one of our cells can survive by maintaining a stable internal composition. Here, enzymes, biochemical catalysts that accelerate the reactions occurring within living organisms, play an essential role..
- Enzymes act on substrates, which are converted into products in one or more steps. Although the functionality of these organic molecules is widely discussed, it is interesting to know that there is more than one type of enzyme, each with its own chemical and functional particularities. Here we tell you all about apoenzymes
What is an enzyme?.
According to the Oxford Languages dictionary,
an enzyme is defined as a soluble protein produced by the cells of the organism, which promotes and regulates chemical reactions in living beings.Although these molecules are mostly of a protein nature, we cannot forget that there are others produced from RNA, the ribozymes, whose particularities we will leave for another occasion.
Enzymes are biocatalysts
and we must stop briefly at this term in order to continue with the concept that concerns us here. A chemical reaction can be observed from both a thermodynamic and a kinetic point of view but, either way, the most immediate is to indicate the change in free energy that occurs as the reaction proceeds.
- A + B → C + D
- For A + B to be transformed into the products, there must be an activation energy, i.e., the minimum amount of energy that a system needs before it can initiate a certain process, that "barrier" that must be overcome to reach the activation state (A.E.). Biocatalysts such as enzymes reduce this energy necessary for these reactions to occur by two mechanisms:
By attaching to the substrate (the starting substance), which weakens its chemical bonds and facilitates their breakage to give rise to the products. By attracting reactive compounds to its surface, which facilitates the overall process.Thus, enzymes act with both specificity and specificity,
enzymes act with both substrate specificity and action specificity to know to whom to bind or what type of reaction to promote, respectively.
. It is clear that we have presented the term to you in a summarized form, as the structure and functionality of enzymes provides enough information to write several books on the subject.
What is an apoenzyme? Once we have briefly circumscribed the term enzyme, we are ready to address the one that concerns us here. Enzymes are usually globular proteins composed of a concatenation of amino acids (almost all enzymes are larger than the substrates but only 3-4 specific amino acids are involved in catalysis) soluble in water but, depending on their composition, two types can be distinguished..
The enzymes in use are composed of one or more protein chains, also known as polypeptide chains. On the other hand,
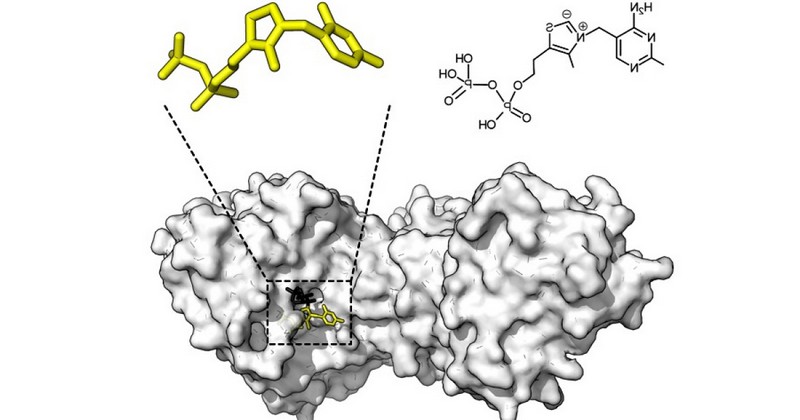
Holoenzymes are chemically more complex, since they are made up of a protein part called apoenzyme and a non-protein part called cofactor.
Holoenzyme= Apoenzyme + cofactor
Let us look at each of these components in detail below. ApoenzymeThus,
we can define an apoenzyme as the protein part of an enzyme (holoenzyme) which, in order to be active, needs to be bound to the corresponding cofactor also known as coenzyme when it is an organic cofactor of a non-protein nature. It is also called apoprotein.:
- The apoenzyme, like the enzymes themselves, is a globular protein uniquely formed by an ordered sequence of amino acids, its simplest subunits. These amino acids perform the enzymatic capacity of the biomolecule but, as we have said, only a few are involved in catalysis themselves.
- Depending on the function they perform, we can distinguish 4 types of amino acids in the apoenzyme
- Non-essential: they are not involved in catalysis per se, but are part of the structure of the apoenzyme. If they are removed, the apoenzyme does not lose its catalytic capacity.
- Structural: they are responsible for the three-dimensional structure of the apoenzyme.
Binding: they establish volatile bonds with the substrate and orient it so that the reaction can be carried out.Catalytic: the 3 or 4 amino acids of the whole structure with the actual catalytic function. They bind to the substrate by a covalent bond and weaken its structure, making the reaction easier.
These last two types of amino acids, catalytic and binding, form the active site, i.e. the area of the enzyme where the substrate binds to be catalyzed.
- . The enzyme-substrate coupling is such that Hermann Emil Fischer, a renowned 20th century German chemist, described such a bond as follows: "the substrate fits the active or catalytic center of an enzyme like a key to a lock". Thanks to this simile, the key-lock complex has historically been used to explain enzyme action in schools and institutes, although in reality it is a much more versatile and adaptable mechanism.
2. CofactorTo fully understand the holoenzyme (and therefore the apoenzyme) it is necessary to describe the cofactor, the non-protein component of the holoenzyme.
Cofactors are basically of two types: metal ions and organic molecules, also known as enzymes.
. Within the group of metal ions we find representatives such as Fe2+, Cu2+, K+, Mn2+, Mg2+ and many others. Mainly, these ions usually act as the catalytic center itself or as stabilizing agents of the holoenzyme conformation. On the other hand, coenzymes are non-protein organic cofactors (if they were composed of amino acids, they would be part of the enzyme chain itself). They are thermostable Biological compounds that, when bound to the apoenzyme, make up the holoenzyme as a whole. It should be noted that coenzyme-apoenzyme binding is not specific, since these organic cofactors can bind to different types of apoenzymes and binding is usually temporary. Some examples of coenzymes are FAD (flavin adenine dinucleotide), Coenzyme A and Coenzyme Q. Surely some of them ring a bell, don't they?:
- Finally, we would like to emphasize that
- the basic mechanism of action of coenzymes can be summarized in the following points
- The coenzyme binds to the apoenzyme, forming the functional holoenzyme.
- The enzyme captures its specific substrate, i.e. the "base" that will give rise to the desired products after the metabolic reaction.
- The holoenzyme attacks the substrate, resulting in a compound with weak bonds that eventually gives rise to an unstable substance.
The enzyme gives up electrons from the substrate to the coenzyme to form this unstable compound. The coenzyme accepts these electrons and detaches from the apoenzyme and travels to "give up" these electrons, thus returning to its initial state.Of course, these steps are laid out in the most reductionist way possible, but the general idea is clear:
an apoenzyme and the cofactor, whether organic or inorganic, give rise to a holoenzyme, a biocatalyst that leads to the formation of a holoenzyme.
The biocatalyst that enables metabolic reactions in our body to happen faster.
General summary
Thus, we can summarize that the apoenzyme is the protein part of a holoenzyme, which accounts for most of its three-dimensional chemical structure. In general, enzymes can be conceived as essential biomolecules for life, since due to their specificity, reversibility, efficiency, great catalytic power and permanence in time, they are capable of accelerating multiple chemical reactions that, without them, would be much slower and more costly. With all this terminological conglomerate we want to emphasize that, in order to know the functionality of a molecule, it is necessary to know also about its chemical structure and components necessary for its functioning. Without the apoenzyme, the concept of a complex enzyme formed by compounds beyond proteins could not be understood.
- Bibliographical references:
- Aguado Esteban, C. (2008). Research in specific therapies for inherited metabolic mutations: cofactor response and antisense therapy.
- Briceño, K. Apoenzyme: Characteristics, Functions and Examples.
- Briceño, K. Holoenzyme: Characteristics, Functions and Examples.
- Monguí Aponte, L. Y., Hernández Guzmán, T. D., & González Gómez, L. F. (2020). Teaching learning of the concepts coenzyme and apoenzyme associated with the study of enzymatic activity: a look from the problem-based learning model using the flipped classroom methodology.
- Moreno, J. C. V. (2016). Pyridoxal phosphate: mechanism of inhibition role as a cofactor and synthesis (Doctoral dissertation, Universidad Complutense).
- Rincón, L. E. C., & Muñoz, L. M. M. (2005). Soil enzymes: indicators of health and quality. Acta Biológica Colombiana, 10(1), 5-18.
(Updated at Apr 14 / 2024)