Disulfuric acid: characteristics and functions of this substance
A summary of the characteristics of oleum, also called disulfuric acid.
We have all heard of sulfuric acid at one time or another, even if only in passing.. This extremely corrosive and dangerous substance is the most widely produced chemical compound in the world. It is particularly important in the petroleum industry, in steel processing, in the manufacture of explosives, detergents and plastics, and in the synthesis of fertilizers.
Without sulfuric acid, it would not be possible to drive the wood and paper industry, many textile mill processes or the production of batteries. Nor can we forget its role in the chemical industry, since it is necessary for the synthesis of other acids and sulfates that are vital for certain processes.
In other words, society would not be as we know it without sulfuric acid, as it plays essential roles in the chemical industry, in the oil industry and in the agricultural field, among many other things. However, not all sulfur compounds are equally well known. Here we turn our attention to one that is much less familiar to the general population: disulfuric acid..
What is disulfuric acid?
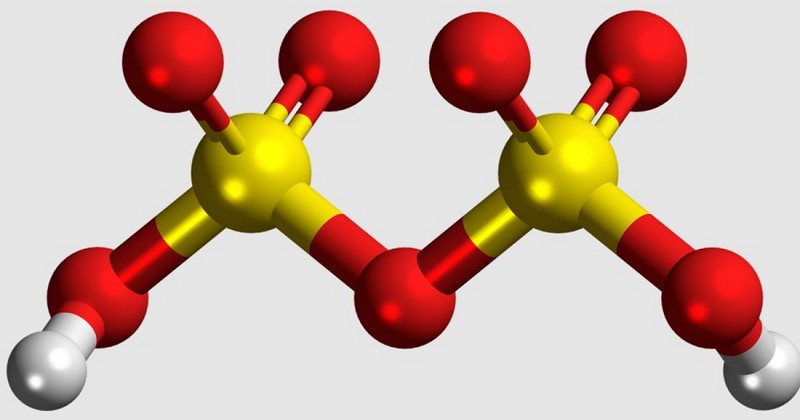
Disulfuric acid, also known as pyrosulfuric acid or oleum, is an oxyacid of sulfur.. The term "oxacid" refers to any acid containing oxygen in its composition, especially those with at least one hydrogen atom (H), one oxygen atom (O) and a variable element, whether it is X, in their chemical structure.
Disulfuric acid is the main component of fuming sulfuric acid or oleum, whose properties and uses we will explain in future sections. For now, its chemical structure is H2SO7. This means that it is made up of two hydrogen atoms (H2), seven oxygen atoms (O7) and two sulfur atoms (S2). The usual sulfuric acid, on the other hand, has two hydrogen atoms, one sulfur atom and four oxygen atoms (H2SO4).
It is also interesting to note that this acid has a molar mass of 178.13 g-mol-1 and that its melting point is 36 degrees, the temperature that marks the change from a solid to a liquid state. There are other acids with the same formula "H2O-(SO3)x", although these cannot be isolated under experimental conditions to date.
Characteristics of oleum
As we have said, disulfuric acid is the main component of fuming sulfuric acid, also known as oleum.. The formula of this solution is ySO3-H2O, where "y" is the total molar mass of the sulfur trioxide part (SO3). However, it can also be designated by the nomenclature "H2SO4-xSO3", where "x" refers to the molar free sulfur trioxide content. When x=1 and y=2, the formula H2S2O7 is obtained, or, in other words, that of disulfuric acid.
It may sound complex, but keep in mind the following idea: an oleum solution can have different properties depending on the percentage concentration of sulfuric acid, and the aforementioned conformation gives rise to disulfuric acid, which is present in solid form up to 36 degrees Celsius at room temperature. As interesting as this whole conglomerate may sound on a chemical level, it should be noted, however, that Disulfuric acid is rarely used in laboratory environments or industrial processes..
Oleum is synthesized by a "contact process", where oxygen groups are added to sulfur (S+O3, SO3) and then dissolved in a sulfuric acid concentrate (H2SO4). Perhaps a chemist would pull his hair out to see such a great oversimplification, but for informative purposes, we take the license to show you the union of both concepts:
Thus, the typical oleum or disulfuric acid is constructed from sulfur to which oxygen and sulfuric acid concentrate have been added. In the world of chemistry, in the end everything is also a matter of mathematical knowledge.
Functions and uses of this substance
Once we have dissected the chemical nature of this complex solution, we can explore its uses, albeit briefly.
1. Production of sulfuric acid
Counter-intuitive as it may sound, a solution that requires sulfuric acid for its synthesis can be useful for the production of sulfuric acid itself..
Because of its high enthalpy of hydration (change in enthalpy when one mole of ions dissolves in enough water to give a dilute solution), oleum can be diluted in water to produce additional concentrated sulfuric acid.
Conversely, if SO3 were added directly to water, a gaseous film of sulfuric acid would form that would be very difficult to handle.
@image( 28510)
2. Transport intermediate
Since oleum is in a solid state up to 36 degrees Celsius, it can be useful for the transport of sulfuric acid, it can be useful for transporting sulfuric acid in tank trucks between oil refineries and various industries.. Once it reaches its destination, oleum can be transformed back to its liquid state. However, this process must be carried out very carefully, as overheating of the material exceeds the safety limits.
In addition, oleum or disulfuric acid is less corrosive than sulfuric acid when in contact with metals, since there are no free water molecules that can attack these surfaces. For this reason, liquid disulfuric acid is also sometimes synthesized for transport between complex pipelines. Due to its ability to "revert" to concentrated sulfuric acid and its ease of changing from solid to liquid state, oleum has many uses in the field of transportation.
3. Disulfuric acid in the explosives industry
Oleum is also used in the is also used in the synthesis of explosives, with the notable exception of nitrocellulose.. This is because solutions of nitric acid (NO3) and sulfuric acid (H2SO4, which may or may not be obtained using disulfuric acid as a base) contain significant amounts of water, which makes them of little use in many explosives manufacturing processes.
4. Use in the study of organic chemistry
Oleum is an aggressive and highly corrosive reactive agent, useful as an intermediate in certain chemical reactions.
Summary
In summary, disulfuric acid can be seen as an intermediate of sulfuric acid, although it is derived from reactions that require it in the first instance. Since it is naturally occurring in solid form, it is suitable for safe transport in many sectors of industry that handle H2SO4 naturally. It is also very interesting to use this compound in its liquid form, since it is less corrosive than sulfuric acid and therefore causes less damage to pipes and other metallic coatings.
Again, we return to the idea that sulfuric acid is essential in the agricultural, lumber, textile, petroleum and many other industries. Thus, although disulfuric acid is not of great use as a compound directly applicable in the laboratory, it does provide some plasticity when transporting, refining and treating sulfuric acid itself.
(Updated at Apr 15 / 2024)